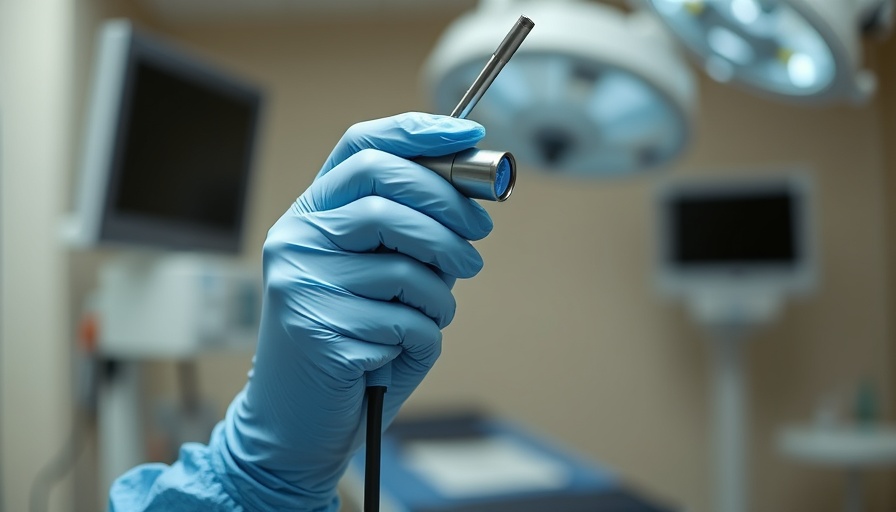
FDA Halts Olympus Medical Device Imports Over Quality Concerns
The Food and Drug Administration (FDA) has recently issued import alerts preventing certain Olympus Medical devices from entering the U.S. The decision comes in response to ongoing concerns regarding the quality systems at an Olympus facility in Japan. In this action, the FDA aims to ensure the safety of patients who depend on these medical devices.
Understanding the Impact on Health and Wellness
This ban affects a total of 58 models of Olympus medical devices that are typically used in various procedures, including those involving the urinary tract, respiratory system, and abdominal and pelvic areas. These models are crucial for performing minimally invasive surgery, which is vital for optimal health and wellness across diverse populations. The alert means that while healthcare providers can continue using existing devices, no new imports will be permitted until quality compliance is achieved.
Patient Safety as a Priority
The import alerts reflect a series of regulatory actions taken by the FDA against Olympus, which has faced criticism for demonstrating a “troubling disregard for patient safety.” The FDA issued three warning letters to Olympus between November 2022 and March 2023, highlighting significant noncompliance issues in their manufacturing processes that heightened the risk of infections associated with reprocessed endoscopes.
The Bigger Picture: Quality in Medical Manufacturing
This latest issue is pivotal not just for Olympus but also for the broader health care community. As we delve into the implications of the FDA's decision, it’s essential to consider what this means for the future of medical device manufacturing. The inconsistent quality of medical devices can jeopardize community health and wellness outcomes. Ensuring that companies adhere to strict quality standards is paramount for maintaining public trust in healthcare systems.
Alternative Solutions and Community Health Initiatives
Amid these challenges, communities must also explore alternative health and wellness solutions to mitigate risks associated with possible disruptions in medical device supplies. This could include emphasizing the importance of natural and complementary medicine practices, such as naturopathy, which focuses on treating health issues through natural means. By diversifying health care approaches, communities can foster resilience and support optimal health in various ways.
What’s Next for Olympus?
As the FDA continues to monitor Olympus’s compliance with its regulations, it's critical for the company to take the necessary corrective actions. Without addressing these quality failures, Olympus risks the potential for extended import bans and further reputational damage. This situation serves as a reminder of the importance of maintaining high manufacturing standards that prioritize patient safety above all.
Final Thoughts: The Call for Vigilance and Action
The ongoing issues surrounding Olympus Medical devices underscore the necessity for vigilance in the health and wellness industry. Stakeholders, including manufacturers, healthcare providers, and patients, must stay informed about regulatory changes that could affect the availability and safety of medical products. It is imperative that everyone involved advocates for better practices to ensure health and wellness in our communities. By doing so, we empower ourselves and those around us to live healthier, longer lives.
 Add Element
Add Element  Add Row
Add Row 



Write A Comment